
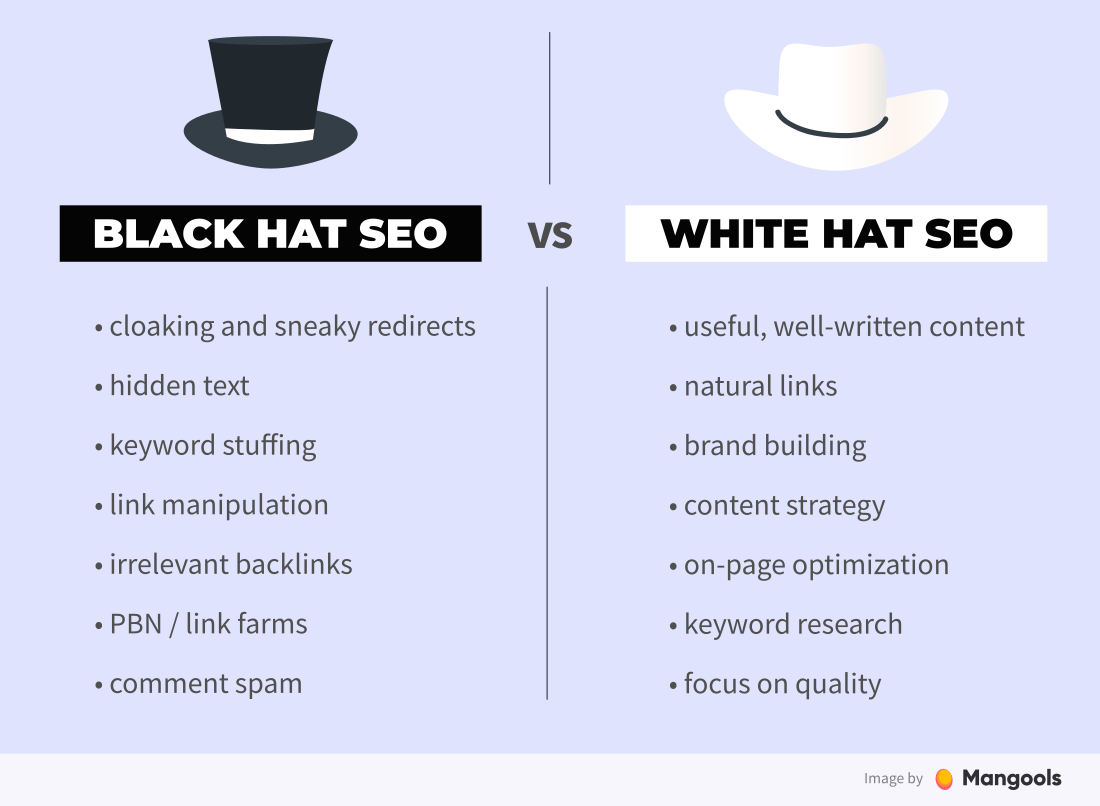
This paper illustrates how functional DOE is an extremely robust and easy-to-use technique for optimizing to a target dissolution profile rapidly with fewer resources.ĭissolution data is collected over time during development and in-depth analyses are required to understand the effect of the formulation/process variables on the product performance. For generic (ANDA) products, the primary objective is to match critical quality attributes (including dissolution over time) to the reference listed drug (RLD). The key determinants contributing to an adverse reaction from the optimal model are age, number of symptoms, period between vaccination onset, sex, state, type of vaccine, how the vaccine was administered, symptoms, history of dementia, and history of chronic obstructive pulmonary disease.įormulation scientists put forth significant hours of work attempting to find an extended release formulation that matches drug release targets. The best fit model is a logistic regression model for ordinal target variables.
#SMOOTHING PARAMETER JMP 9 GRAPH BUILDER PRO#
The predictive model is build using JMP Pro 16 and SAS Enterprise Miner 14.1, using logistic regression and decision tree with both binary document term matrix and term frequency inverse document frequency, with the model evaluation based on lowest misclassification area. Next, unstructured data in the from of text describing symptoms, medical history, medication and allergies are converted into a document term matrix and then combined with the structured variables to build a model to predict for the severity of the adverse reaction. The severity of an adverse reaction is first derived from the variables describing the vaccine recipient outcome following a reaction. This paper uses the combination of both structured and unstructured variables from VAERS to model the adverse reactions of COVID-19 vaccines. Data from the United States Vaccine Adverse Event Reporting System, VAERS, has the potential to help determine if the safety concerns of the vaccines are founded. However, the unprecedent rate at which they were developed and administered had raised doubts in the community regarding their safety. The COVID-19 vaccines are crucial to ending the global pandemic that has caused surges of infections and deaths globally. DOEs in retirement are simpler, focusing on things like optimizing an ice cream recipe and reducing the amount of time to produce maple syrup on a conventional stove. We have had more than one case where DSDs identified antagonistic interactions early enough to avoid program delays. When Bradley Jones and Christopher Nachtsheim introduced definitive screening experiments in 2011, I had moved on to Bausch + Lomb. We could run smaller experiments and still get the necessary data, which was very important in the waning days of the Kodak Research Labs when strict quotas were placed on the number and size of experiments. Experiments didn’t have to be orthogonal. In the late 1990s when JMP added optimal designs, we had some new tools. In the 1990s we had JMP, but our DOEs were still basically catalog designs. In the 1980s we started using mainframe SAS. As computer tools progressed, we moved from pencil and paper to in-house mainframe programs. After successfully optimizing this solution that had two statistically significant three-way interactions, I was hooked on designed experiments. Like most young engineers at Kodak, I started out running one-factor-at-a-time designs, until we needed to optimize a five-component solution.


 0 kommentar(er)
0 kommentar(er)
